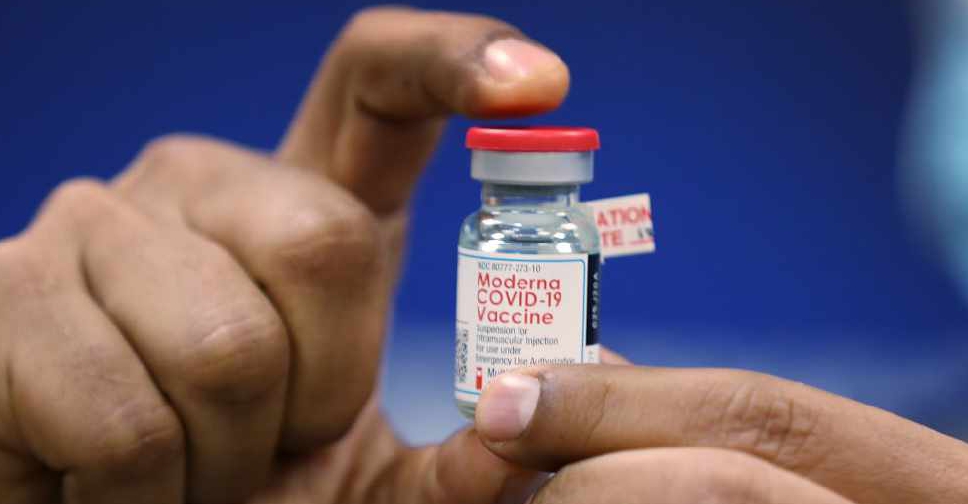
Europe's drug regulator gave the green light to Moderna Inc's COVID-19 vaccine on Wednesday as authorities accelerate inoculation efforts amid fears about more infectious variants of the coronavirus.
Vaccinating the European Union's 450 million people could be crucial to ending a pandemic that has killed almost 1.9 million people globally.
The approval comes as countries are racing to contain two variants found in South Africa and Britain that are more transmissible and have driven a surge in infections.
The EU's European Medicines Agency (EMA) said it had given the go-ahead for the use of the Moderna vaccine on people aged over 18 following an assessment of the data on quality, safety and efficacy.
The final step before it can be rolled out across the European Union is approval by the bloc's executive body, the European Commission, which is expected to follow soon.
The decision, coming just over a year since the first outbreak of the virus was identified in China.
The EU earlier approved a shot from Pfizer Inc and BioNTech SE, also based on mRNA technology.
The regulator has given a conditional marketing approval for both, rather than the ultra-fast emergency use clearance issued by Britain, which the EMA says requires more detailed study of the data.
The EU has ordered 160 million doses of the shot developed by Moderna, a U.S. biotech company, enough to vaccinate 80 million people in its 27 member states.
The vaccine was about 95% effective at preventing illness in clinical trials, which found no serious safety issues. It has to be stored and shipped frozen, but does not require the ultra-cold temperatures of the Pfizer/BioNTech vaccine. Once thawed, it can be kept at typical refrigerator temperatures.


 At least 24 dead in Texas flash flooding
At least 24 dead in Texas flash flooding
 Aid foundation says two of its workers injured in Gaza
Aid foundation says two of its workers injured in Gaza
 Hamas says it responds to Gaza ceasefire proposal in 'positive spirit'
Hamas says it responds to Gaza ceasefire proposal in 'positive spirit'
 Russia pounds Kyiv with largest drone attack, hours after Trump-Putin call
Russia pounds Kyiv with largest drone attack, hours after Trump-Putin call
 Trump says he expects Hamas decision in 24 hours on 'final' peace proposal
Trump says he expects Hamas decision in 24 hours on 'final' peace proposal




