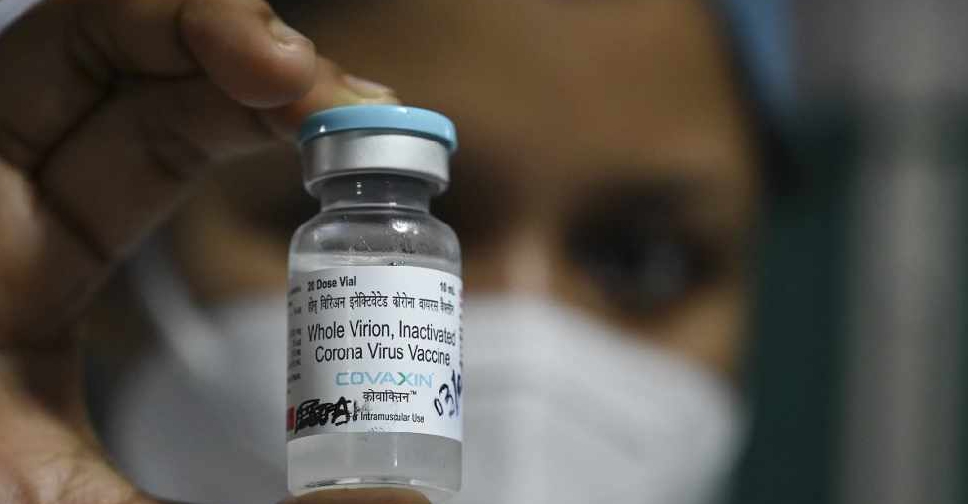
Bharat Biotech's homegrown COVID-19 vaccine has shown an interim vaccine efficacy of 81% in late-stage clinical trials, the Indian company said on Wednesday.
The interim analysis was based on 43 recorded cases of COVID-19 in the trial of 25,800 participants, conducted in partnership with the Indian government's medical research body.
Thirty-six of the 43 cases were recorded in participants who received a placebo, compared with seven cases in people who were given the Bharat Biotech vaccine, pointing to an efficacy rate of 80.6%, the company said.
India had approved the vaccine, branded COVAXIN, in January without late-stage efficacy data, raising questions about its effectiveness.
India's vaccination drive, currently underway, includes COVAXIN and a vaccine developed by Oxford University and AstraZeneca.
Earlier this week, Indian Prime Minister Narendra Modi was inoculated with the first dose of COVAXIN.


 UK government sparks confusion over 'pause' on Chagos Islands deal
UK government sparks confusion over 'pause' on Chagos Islands deal
 Swiss government to pay $56,000 to each victim of Crans-Montana bar fire
Swiss government to pay $56,000 to each victim of Crans-Montana bar fire
 Arab League condemns Israeli settlers' attack on West Bank mosque
Arab League condemns Israeli settlers' attack on West Bank mosque
 Death toll from Brazil floods rises, dozens still missing
Death toll from Brazil floods rises, dozens still missing
 India's Modi to address Israel's Knesset during Israel visit
India's Modi to address Israel's Knesset during Israel visit




